Molecular Nature of Matter
Molecular Nature of Matter: Overview
This topic explains the molecular nature of matter. It also discusses the atomic hypothesis in ancient India and Greece and describes the mean free path with illustration here.
Important Questions on Molecular Nature of Matter
of a gas at a pressure of is compressed to . Taking the temperature to remain constant, the increase in pressure, is
Under what conditions will a pure sample of an ideal gas not only exhibit a pressure of but also a concentration of ?
Select one correct statement. In the gas equation, PV = nRT
If are pressure, volume, molar mass, temperature and gas constant respectively, then for an ideal gas, the density is given by
The volume of air in a car tyre is about at a temperature of and pressure .
Calculate the number of molecules in the tyre( in no. of Molecules).
A container of an ideal gas that is isolated from its surroundings is divided into two parts. One part has double the volume of the other. The pressure in each part is and the temperature is the same. The partition is removed. What is the pressure in the container now?
Avogadro’s law, a statement that under the same conditions of temperature and pressure, equal volumes of different gases contain an equal number of _____.
The force of interaction between two molecules of a gas is very large compare to the force interaction between the molecules of solid.
Define the terms:
(1) Gram mole and kilogram mole.
(2) Most probable speed.
(3) Mean speed or probable speed.
(4) Root mean square speed
What are the main characteristics of a gaseous state?
All the real gases follow the equation, at all conditions of temperatures and pressures.
Which one is the correct dimensional formula for Boltzmann constant?
Boyle's law states that when the temperature of a gas is kept constant, the volume of a fixed mass of gas is inversely proportional to its pressure.
When gases react together, their reaction volume bears a simple ratio to each other, under the same conditions of temperature and pressure. This law was proposed by _____.
moles of an ideal diatomic gas with initial temperature is compressed from to . The thermodynamic process is such that , where is a constant. Then, the value of is close to (the gas constant, ).
The internal energy of a gram-molecule of an ideal gas depends on _______.
For next two question please follow the same
The figure shows a V - T graph of process carried on one mole of mono atomic gas. The slope of the graph in the process CA varies as where is a constant and p is the pressure of the gas.
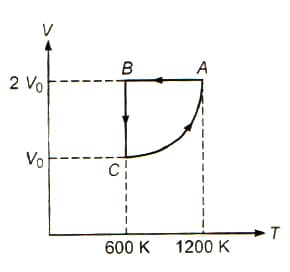
The relation between temperature and pressure variation for the process CA is
The average intermolecular distance is considerably small as compared to the diameter of the molecule.
